Scroll to:
Natural Sorbent Based on Zeolite-Containing Rocks of Tatarsko-Shatrashan Deposit for Treatment of Surface and Waste Water from Organic Pollutants
https://doi.org/10.23947/2541-9129-2025-9-2-112-120
EDN: LJIZLA
Abstract
Introduction. The modern industrial and environmental challenges require the identification of optimal sorbents for water purification from organic contaminants. Sorbents like activated carbon and silicon dioxide have been widely used, but the problem of selecting the optimal sorbent that can adequately purify water remains relevant. There is information in the scientific literature about potential use of sorbents from natural zeolite-containing minerals for this purpose. However, this approach is not well developed; the materials are poorly studied and, as a result, are rarely used to solve environmental problems. This work aims to fill this gap by studying the sorption properties of a natural sorbent based on zeolite-containing rocks of the Tatarsko-Shatrashan deposit.
Materials and Methods. The method of ascending liquid column chromatography became the basis for this study. The sorption material was loaded into a chromatographic column with a length of 120 mm and an inner diameter of 3 mm. The model organic substances in the vial moved along the length of the sorption layer. Trichloroethane, ethyl acetate, methyl ethyl ketone, dichloroethane, and trichloroethylene were used as model compounds. We summarized the significant data in tables and visually represented it in graphs.
Results. The technological characteristics of natural sorbents obtained on the basis of zeolite-containing rocks of the Tatarsko-Shatrashan deposit have been experimentally investigated. The absolute retention time of the studied sorbates, as well as their sorption capacity in relation to zeolite-containing rocks of the Tatarsko-Shatrashansky deposit, were determined. The dependence of the retention time of model organic substances on the length of the sorption layer, which was determined by the physical-chemical nature of the sorbate under study, has been established. From the same point of view (as components of the dependence), the boiling points of model organic substances, dipole moments, refractive indices, and densities were considered. The experimental data were statistically processed, and the absolute and relative errors of a single measurement were determined. All sorbates considered in the framework of this scientific work showed significant or high sorption capacity. The recorded minimum was 34% (methyl ethyl ketone); the maximum was 72% (ethyl acetate). At the same time, ethyl acetate had an extremely short retention time in a 10-centimeter sorption layer (26 min). The longest retention time was for methyl ethyl ketone (314 min). Its sorption capacity was minimal (34%).
Discussion and Conclusion. The prospects of the studied material for the purification of surface and waste water from major pollutants in the natural environment have been experimentally proven. It has been determined that zeolite-containing rocks from the Tatarsko-Shatrashan deposit can adsorb 34–72% of organic compounds that pollute water. They can be used in technological processes for the purification of natural and wastewater from major environmental pollutants.
Keywords
For citations:
Taneeva A.V., Snigireva Yu.V., Khizbullin R.N., Shlykova D.A., Novikov V.F. Natural Sorbent Based on Zeolite-Containing Rocks of Tatarsko-Shatrashan Deposit for Treatment of Surface and Waste Water from Organic Pollutants. Safety of Technogenic and Natural Systems. 2025;(2):112-120. https://doi.org/10.23947/2541-9129-2025-9-2-112-120. EDN: LJIZLA
Introduction. One of the conditions for environmental safety is the protection of water bodies from anthropogenic and natural pollution. A special case of this problem is the negative impact of organic compounds such as petroleum products, phenols, aromatic hydrocarbons, and organochlorine [1]. These pollutants spread relatively quickly in water over considerable distances and harm the environment even in neighboring regions, which can lead to irreversible changes in the ecosystem [2]. Phenol and its derivatives are especially dangerous for the environment and humans, as many of them are mutagens and teratogens capable of disrupting the endocrine system [3]. In addition, under certain conditions, the phenol molecule is transformed into compounds with a higher hazard class [4]. Biodegradation of phenol can accelerate in summer, when aerobic microorganisms intensively oxidize organic compounds [5]. The limiting stage of this process is the mass transfer of oxygen molecules from the gas phase to the aqueous phase [6]. In addition to phenols, other classes of organic compounds can be found in surface waters: aromatics and alkylaromatics, alkanes, carboxylic acids, hexachlorane, hexachlorobenzene, benz(a)pyrene, acetatanaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(g,h,i)perylene, indeno(1,2, 3-cd)pyrene [7].
Source [8] provides information collected over 15 years on the contamination of organic compounds in the bottom sediments of the Belaya River. Analysis of the ecological status of surface waters in various regions of Russia [9] has revealed that in many cases, water resources do not meet the standards. As a rule, cities rely on surface water for their water supply. Water utilities treat and chlorinate this water [10], which can lead to the formation of organochloride compounds that pollute drinking water. These compounds can be removed through various methods, such as sorption, which uses activated carbon and synthetic zeolite materials to remove toxic impurities from water. Recent studies indicate that natural zeolite minerals have promising potential for this purpose due to their well-developed systems of macro- and micropores that effectively remove organic compounds from water [11]. A system of cavities and channels run through the three-dimensional alumino–silico–oxygen framework of natural zeolites. The huge inner surface and cavernous structure of zeolite-containing rocks ensures strength of adsorption processes, which is sufficient for efficient wastewater treatment by industrial enterprises from toxic impurities. However, natural zeolite rocks are not well studied, which hinders their widespread use in solving environmental problems [12].
The aim of this study is to experimentally investigate the technological properties of natural sorbents based on zeolite-containing rocks of the Tatarsko-Shatrashansky deposit.
Materials and Methods. A natural sorbent based on zeolite-containing rocks from the Tatarsko-Shatrashansky deposit has been studied (Table 1).
Table 1
Chemical composition of the natural sorbent [12]
|
Elements |
% wt |
|
Silicon dioxide SiO2 |
66.00 |
|
Titanium dioxide TiO2 |
0.35 |
|
Aluminum oxide Al2O3 |
6.19 |
|
Iron oxide Fe2O |
2.65 |
|
Manganese oxide MnO |
0.01 |
|
Calcium oxide CaO |
17.00 |
|
Magnesium oxide MgO |
1.45 |
|
Sodium oxide Na2O |
0.16 |
|
Potassium oxide K2O |
1.43 |
|
Phosphorus oxide P2O5 |
0.13 |
The natural elements under consideration are thermally stable and acid resistant. The total cation exchange
capacity — 130.0 mg-eq / 100 g. Calcium plays a major role in the metabolic process. Table 2 provides the main characteristics of the material under study.
Table 2
Characteristics of the natural sorbent under study
|
Indicators |
Physical and mechanical properties |
|
Appearance |
Granules of light gray or white color |
|
Porosity |
37.25–55.72% |
|
Density |
2.03–2.37 g/cm³ |
|
Mechanical crushing strength |
At 200 °С — 46 kg/cm², at 2500 °С — 59 kg/cm² |
|
Vibration wear |
0.96% |
|
Bulk weight |
0.4–1.2 g/cm³ |
|
Volume weight |
1.10 g/cm³ |
|
Effective pore diameter |
0.4 Nm (4А°) |
|
Thermal resistance |
Higher than 450°С |
|
Solubility in water |
Insoluble |
To prepare the sorbent, rocks containing zeolite from the Tatarsko-Shatrashansky deposit were mechanically activated. They were crushed in a ball mill; the fractions were separated, and then treated with a 1:1 solution of hydrochloric acid. Next, the resulting material was washed with water until a neutral reaction was achieved and subjected to heat treatment at 450–500°C for 5 hours. The sorbent obtained was placed in 150 mm long glass chromatographic columns with an inner diameter of 3 mm. Vials containing the model liquids under study, organic substances with various physical and chemical properties, were attached to the bottom of the columns. As the model organic substances passed through the sorption layer of the zeolite-containing rock, their movement up through the channels and pores was recorded. The time of passage was measured every 10 millimeters of the sorbent and kinetic curves were plotted for the dependence of the retention of model organic substances on the height of the sorption layer.
Sorption properties were determined by the formula:
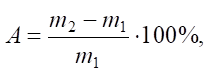 (1)
(1)
where A — sorption capacity of the material under study, %; m1 — mass of the initial adsorbent; m2 — mass of the adsorbent saturated with an organic solvent.
For statistical processing of experimental data, the absolute and relative errors of a single measurement were determined with a confidence probability of 0.95. Table 3 provides the results for hexane sorption.
Table 3
Absolute and relative errors in the measurement of hexane on the tested sorbents
|
Measurement criteria |
Error |
|
|
absolute, ∆ |
relative, δ % |
|
|
5 |
7.16 |
13.64 |
|
10 |
6.40 |
12.12 |
|
20 |
5.29 |
11.96 |
|
30 |
5.15 |
10.78 |
Results. Table 4 provides information on physical-chemical properties of model organic substances (sorbates).
Table 4
Characteristics of model sorbates*
|
Model sorbates |
Formula |
Tкип |
d |
nD²⁰ |
t10 |
A |
μ |
|
Trichlorethane |
CHCl3 |
74 |
1.453 |
1.4463 |
152 |
45 |
1.15 |
|
Ethyl acetate |
C4H8O2 |
77.1 |
0.900 |
1.3720 |
26 |
72 |
2.48 |
|
Methyl ethyl ketone |
C4H8O |
79.6 |
0.805 |
1.3800 |
314 |
34 |
2.84 |
|
Dichloroethane |
C2H4Cl2 |
83.5 |
1.253 |
1.4400 |
61 |
62 |
1.80 |
|
Trichloroethylene |
C2HCl3 |
87.2 |
1.464 |
1.4800 |
88 |
56 |
0.85 |
|
Hexane |
С6Н14 |
68.0 |
0.660 |
1.416 |
59 |
46 |
0.05 |
where *d — density, g/cm³; Tкип — boiling point, °С; nD²⁰ — refractive indices at 20°С; t10 — retention time of model organic substances in a 10-centimeter sorption layer, min; A — sorption capacity, %; μ — dipole moment, D
As a result of the experiments (Table 4) the retention time of ethyl acetate was extremely low (26 min). Trichloroethane, which had a lower boiling point (74°C), was washed out of the column later than ethyl acetate with a boiling point of 77.1°C. Obviously, this could be explained by the higher molecular weight of trichloroethane (M = 119.4 g/mol) compared to ethyl acetate (M = 88.11 g/mol). In this case, the order in which the components exit was not determined by their boiling points. This dependence was more complicated. It took into account the chemical nature of the model organic substances used, as well as the possibility of their adsorption and desorption by the pores of the material under study. At the same time, despite the low retention time of ethyl acetate, its sorption capacity was relatively high (72%) compared to other organic substances studied. The sorption capacity of all the studied organic substances was quite high (34–72%), which made it possible to use these materials to purify water from organic compounds.
To evaluate the kinetic characteristics of the sorption process, dependencies were constructed linking the retention time of model sorbates and the height of the sorption layer in the ranges from 0 to 5 cm (Fig. 1) and from 5 to 10 cm (Fig. 2).
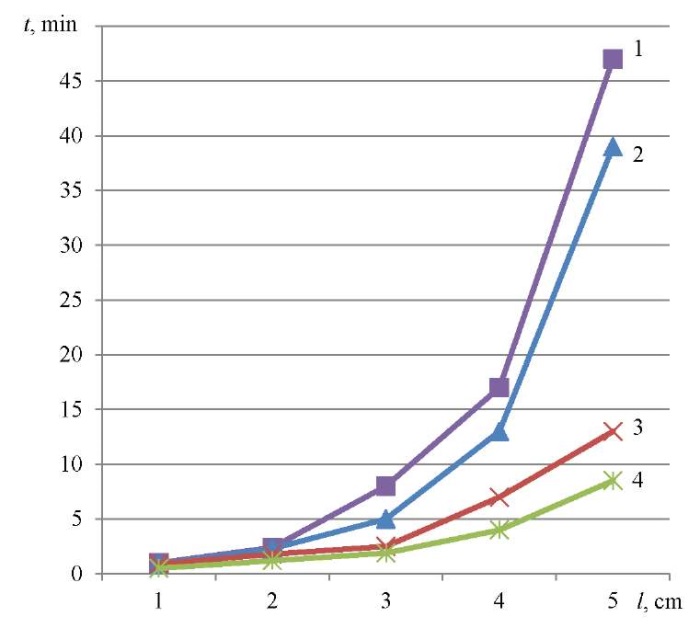
Fig. 1. The effect of retention of model sorbates on the nature of sorption processes (sorbent layer from 0 to 5 cm):
1 — methyl ethyl ketone; 2 — trichloroethane; 3 — trichloroethylene; 4 — dichloroethane
As it can be seen from Figure 1, the dependence had a parabolic character. Obviously, this was due to the fact that at the initial moment of time (up to 5 mm of the sorption layer) there was no equilibrium between the organic liquid and the solid. It was established after sorption, and an almost linear pattern was observed at a distance of more than 5 cm. The exception was methyl ethyl ketone, which had a sufficiently high value of the dipole moment (μ = 2.84D)
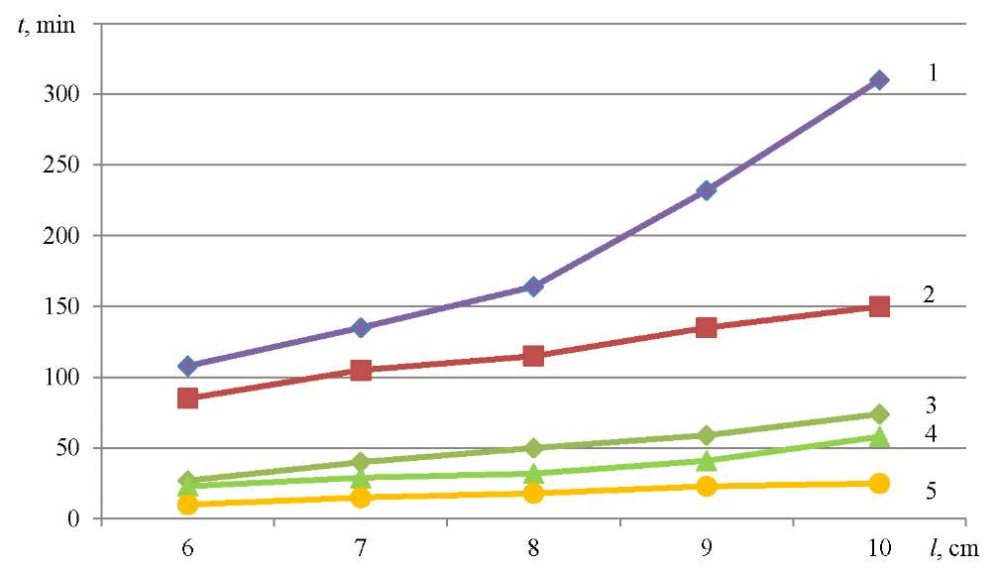
Fig. 2. Dependence of the retention time of model organic substances on the height of the sorption layer of zeolite-containing rock (from 5 to 10 cm):
1 — methyl ethyl ketone; 2 — trichloroethane; 3 — trichloroethylene; 4 — dichloroethane; 5 — ethyl acetate
It should be noted that for all the organochlorine compounds studied (trichloroethane, dichloroethane, trichloroethylene), the angles of inclination of straight lines were almost identical (Fig. 2). This indicated the additivity of the sorption process for organochlorine compounds.
An important parameter of an organic molecule is the dipole moment. It characterizes the asymmetry of charge distribution in an electrically neutral molecule, which makes it possible to form electric dipoles with the same charge values +g and –g. The dipole moment of a molecule is determined as a result of vector addition of the dipole moments of individual bonds. The dipole moments of organic compounds characterize the polar properties of a molecule, as well as determine the direction and strength of intermolecular electrostatic interactions and the sorption properties of porous materials.
Figure 3 shows the dependence of the retention time of model sorbates (tуд) on their boiling points (Tкип, °С) and dipole moments (μ, D).
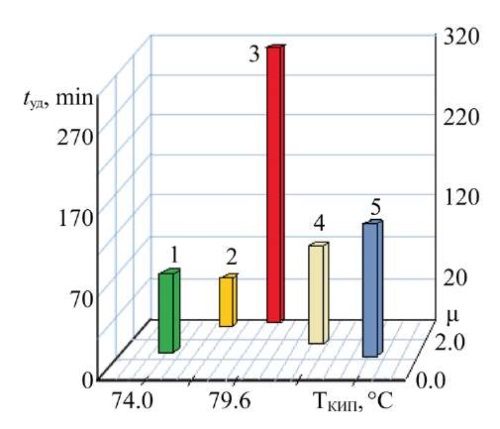
Fig. 3. Dependence of the retention time of model organic substances on their boiling points and dipole moments:
1 — trichloroethane; 2 — ethyl acetate; 3 — methyl ethyl ketone; 4 — dichloroethane; 5 — trichloroethylene
As it can be seen from Figure 3, the release time of ethyl acetate (2) and methyl ethyl ketone (3) differed significantly, although they had similar values of dipole moments and boiling points. Methyl ethyl ketone retained significantly longer (tуд = 314 min) than ethyl acetate (tуд = 26 min), dichloroethane (tуд = 61 min), trichloroethylene (tуд = 88 min), and trichloroethane (tуд = 152 min). Let us note that according to the Rorschneider classification in the sorbate—sorbent system, methyl ethyl ketone determines the dispersion interaction. Obviously, the Van der Waals forces play a leading role in this process, and the sorption time is more significant. This is also due to the fact that methyl ethyl ketone is characterized by a higher polarity (dipole moment — μ = 2.84D), as well as a higher boiling point (Tкип = 79.6°С).
The refractive index also determines the polarity of the model organic substances. It is related to molecular refraction, and is a measure of the electronic polarizability of the shell of a substance molecule.
Figure 4 shows the dependence of the retention time of the model sorbates on their boiling temperatures (Tкип, °С) and refractive indices (nD²⁰).

Fig. 4. Dependence of the retention time of model organic substances on their boiling points and refractive indices:
1 — trichloroethane; 2 — ethyl acetate; 3 — methyl ethyl ketone; 4 — dichloroethane; 5 — trichloroethylene
As it can be seen from Figure 4, ethyl acetate and methyl ethyl ketone were similar in two parameters:
- boiling points (77.1°С and 79.6°С respectively);
- refraction (1.37 and 1.38 respectively).
Methyl ethyl ketone is characterized by a higher retention time compared to other sorbates studied. Apparently, this is due to the higher energy of the orientational interaction of methyl ethyl ketone with the sorbent surface.
Figure 5 allows us to consider the density of sorbates in relation to retention time and boiling point.
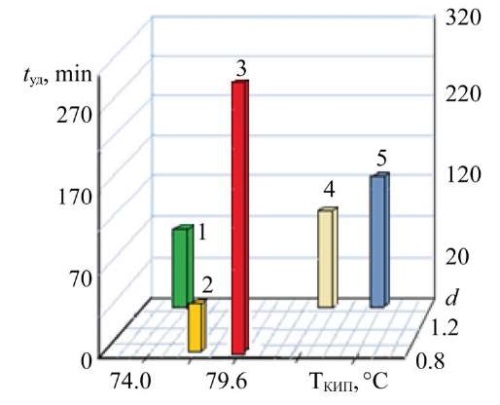
Fig. 5. Dependence of the retention time of model organic substances on their boiling points and density:
1 — trichloroethane; 2 — ethyl acetate; 3 — methyl ethyl ketone; 4 — dichloroethane; 5 — trichloroethylene
Thus, within the framework of this scientific work, it was found that the sorption capacity of the materials under consideration varied from 34% to 72%. Ethyl acetate (C4H8O2) had a particularly high rate (72%). Its other characteristics were: dipole moment — 2,48; retention time of model organic substances in a 10-centimeter sorption layer — 26 min; refractive index at 20°С — 1.3720; boiling point — 77.1°С; density — 0.9 g/cm³. Methyl ethyl ketone was similar to ethyl acetate in terms of boiling point, density, refraction, and dipole moment. However, among the sorbates considered, its sorption capacity was minimal (34%), and the retention time was the longest (314 min).
Discussion and Conclusion. The results of the study indicate that a high-quality sorbent can be produced using zeolite-containing rocks from the Tatarsko-Shatrashansky deposit. This material has the potential to purify water from major organic pollutants. It was found that, the best result in terms of sorption capacity was to remove ethyl acetate if necessary. The sorbent in question takes 72% of this substance from the water. The minimum is associated with methyl ethyl ketone. In this case, the lowest but acceptable sorption capacity (34%) is established.
Thus, the sorption properties of natural zeolites are considered to be good. The findings of this scientific study can be applied in technological processes for water purification from major contaminants.
References
1. Baute-Pérez D, Santana-Mayor Á, Herrera-Herrera AV, Socas-Rodríguez B, Rodríguez-Delgado MA. Analysis of Alkylphenols, Bisphenols and Alkylphenol Ethoxylates in Microbial-Fermented Functional Beverages and Bottled Water: Optimization of a Dispersive Liquid-Liquid Microextraction Protocol Based on Natural Hydrophobic Deep Eutectic Solvents. Food Chemistry. 2021;377:131921. https://doi.org/10.1016/j.foodchem.2021.131921
2. Pradeep NV, Anupama S, Navya K, Shalini HN, Idris M, Hampannavar US. Biological Removal of Phenol from Wastewaters: A Mini Review. Applied Water Science. 2015;5:105–112. https://doi.org/10.1007/s13201-014-0176-8
3. Xiaowen Xie, Xiaoguo Ma, Lihui Guo, Yinming Fan, Guolong Zeng, Mengyuan Zhang, et al. Novel Magnetic Multi-Templates Molecularly Imprinted Polymer for Selective and Rapid Removal and Detection of Alkylphenols in Water. Chemical Engineering Journal. 2019;357:56–65. https://doi.org/10.1016/j.cej.2018.09.080
4. Ighalo JO, Yap PS, Iwuozor KO, Aniagor CO, Tianqi Liu, Dulta K, et al. Adsorption of Persistent Organic Pollutants (POPs) from the Aqueous Environment by Nano-Adsorbents: A Review. Environmental Research Reviews. 2022;212:113123. https://doi.org/10.1016/j.envres.2022.113123
5. Kazakov DA, Vol’khin VV, Borovkova IS, Popova NP. Increase in Speed of Phenol Biodegradation under the Conditions of Mass Transfer Enhancement. Ecology and Industry of Russia. 2014;9:32–35. (In Russ.) https://doi.org/10.18412/1816-0395-2014-9-32-35
6. Quijano G, Rocha-Ríos J, Hernández M, Villaverde S, Revah S, Muñoz R, et al. Determining the Effect of Solid and Liquid Vectors on the Gaseous Interfacial Area and Oxygen Transfer Rates in Two-Phase Partitioning Bioreactors. Journal of Hazardous Materials. 2010;175(1–3):1085–1089. https://doi.org/10.1016/j.jhazmat.2009.10.020
7. Fatyanova EV, Khatmullina RM, Galaktionova EB, Yapparova GR, Safarova VI. Organic Compounds in the Belaya River Bottom Sediments. Life Safety. 2017;11(203):25–30. (In Russ.)
8. Dymnikova OV, Borman AE. Dynamics of Anthropogenic Pollution of the Glubokaya River in the Rostov Region. Safety of Technogenic and Natural Systems. 2022;1:48–56. https://doi.org/10.23947/2541-9129-2022-1-48-56
9. Taneeva AV, Dmitrieva AV, Snigirevа YuV, Novikov VF. Features of the Gas Chromatographic Method for Monitoring the Content of Phenols in an Aqueous Medium. Problems of Contemporary Science and Practice. Vernadsky University. 2023;2(88):7–18. (In Russ.) URL: http://vernadsky.tstu.ru/pdf/2023/02/004.pdf (accessed: 14.01.2025).
10. Somin V, Betz S, Komarova L. The Use of Sorbents Based on Natural Raw Materials for the Purification of Phenol-Containing Waters. Ecology and Industry of Russia. 2016;20(12):14–17. (In Russ.) https://doi.org/10.18412/1816-0395-2016-12-14-17
11. Tatarintseva EA, Bukharova EA, Ol’shanskaya LN. Sorption Material for Water Cleaning from Petroleum Products. Ecology and Industry of Russia. 2014;7:21–28. (In Russ.)
12. Burov IA, Tyurin AN, Yakimov AV, Ishkaev TKh, Izotov VS, Kikilo DA, et al. Zeolite-Bearing Rocks of Tatarstan and Their Application. Kazan: Fen; 2001. 176 p. (In Russ.)
About the Authors
A. V. TaneevaRussian Federation
Alina V. Taneeva, Cand. Sci. (Chemistry), Associate Professor of the Department of Energy Supply of Enterprises, Construction of Buildings and Structures
51, Krasnoselskaya St., Kazan, 420066
Yu. V. Snigireva
Russian Federation
Yuliya V. Snigireva, Postgraduate Student of the Department of Energy Supply of Enterprises, Construction of Buildings and Structures
51, Krasnoselskaya St., Kazan, 420066
R. N. Khizbullin
Russian Federation
Radik N. Khizbullin, Cand.Sci. (Phys.-Math.), Associate Professor of the Electric Stations Named after V.K. Shibanov Departmen
51, Krasnoselskaya St., Kazan, 420066
D. A. Shlykova
Russian Federation
Darya A. Shlykova, Postgraduate Student of the Electric Stations Named after V.K. Shibanov Department
51, Krasnoselskaya St., Kazan, 420066
V. F. Novikov
Russian Federation
Vyacheslav F. Novikov, Dr. Sci. (Chemistry), Professor, Professor of the Department of Energy Supply of Enterprises, Construction of Buildings and Structures
51, Krasnoselskaya St., Kazan, 420066
Review
For citations:
Taneeva A.V., Snigireva Yu.V., Khizbullin R.N., Shlykova D.A., Novikov V.F. Natural Sorbent Based on Zeolite-Containing Rocks of Tatarsko-Shatrashan Deposit for Treatment of Surface and Waste Water from Organic Pollutants. Safety of Technogenic and Natural Systems. 2025;(2):112-120. https://doi.org/10.23947/2541-9129-2025-9-2-112-120. EDN: LJIZLA








































