Scroll to:
Fine Steel Structure after Microarc Molybdenum Steel Saturation
https://doi.org/10.23947/2541-9129-2025-9-3-250-256
EDN: BECKGZ
Abstract
Introduction. In modern production, it is important to increase reliability and durability of steel products. One way to achieve this is by creating high-hardness, wear-resistant coatings on their surface. These coatings can be formed using the method of diffusion saturation, which involves the introduction of carbide-forming elements into the metal. Traditional methods for creating these coatings are time-consuming, taking up to 8 hours or more. To accelerate this process, researchers have proposed using high-energy methods such as laser and plasma treatments. However, these methods require specialized equipment that can be expensive. In this paper, we consider a method for creating a high-hardness molybdenum-based coating by microarc alloying. This method involves exposing a processed steel product immersed in coal powder to multiplicative microarc discharges that occur between the metal surface and the surrounding powder medium. The discharges are generated when an electric current is passed through them. This method allows for a significant increase in the process of diffusive surface saturation. It is characterized by simplicity and low energy consumption. The properties of the resulting coatings are primarily determined by their fine structure. Therefore, studying this structure is a crucial task. The aim of this research was to investigate the features of the fine structure of the steel surface layer after microarc molybdenum plating.
Materials and Methods. A coating containing finely dispersed ammonium molybdate powder and an electrically conductive gel as a binder in a volume ratio of 1:1 was used as a source of molybdenum for diffusion saturation. The coating was applied to the surface of cylindrical samples made of 20 steel with a diameter of 12 mm and a length of 35 mm. Then they were immersed in a metal container with a carbon powder with a particle size 0.4–0.6 mm. An electric current was passed through this powder for 6 minutes, with a surface current density of 0.53 A/cm2. A Neophot-21 microscope, an ARL X'TRA-435 diffractometer, a ZEISS CrossBeam 340 scanning electron microscope with an X-ray microanalyzer, and a NanoEducator scanning probe microscope were used to study the fine structure of steel.
Results. After microarc molybdenum saturation of steel samples, a coating with a multilayer structure and a complex phase composition was formed. On the surface of the material, there was a slightly etched layer with a thickness of 50–55 µm, under which there was a carbonized layer with eutectoid structure and a thickness of approximately 200 µm, and the original ferrite-pearlite structure was preserved lower. The base of the slightly etched layer was a dispersed ferrite-carbide mixture containing about 47% wt. % of Mo and having a microhardness of 8–9 GPa. This layer contained carbide inclusions up to 5 µm in size, containing 94 wt. % of Mo and having microhardness up to 21 GPa. The surface relief was characterized by the presence of carbide inclusions of 3–5 µm in size, as well as multiple nanoscale inclusions protruding above the surface to a height of 10 to 150–200 nm.
Discussion. The results of the study, obtained using metallographic analysis, scanning electron microscopy, X-ray phase analysis and atomic force microscopy, showed that during microarc molybdenum steel saturation, a diffusion layer was formed containing nanoscale particles of the carbide phase. These particles reached a volume fraction of up to 70% and were located at the base of the layer. This layer was a ferrite-carbide eutectoid mixture. A quantitative assessment of the strengthening effect of these particles confirmed that the presence of such particles, characterized by high microhardness, determines the high hardness of the resulting coating.
Conclusion. Microarc molybdenum steel saturation is an effective method for creating coatings with exceptional performance characteristics. These coatings are characterized not only by their high hardness, due to the presence of nanoscale carbide particles located in a ferrite-carbide base, but also by their improved mechanical properties. This makes them promising for use in various industries where high wear resistance and durability of products are required. The research findings indicate that microarc molybdenum steel saturation significantly reduces processing time and avoids the use of expensive equipment, which makes it more affordable for industrial implementation.
Keywords
For citations:
Stepanov M.S., Dombrovskii Yu.M. Fine Steel Structure after Microarc Molybdenum Steel Saturation. Safety of Technogenic and Natural Systems. 2025;9(3):250-256. https://doi.org/10.23947/2541-9129-2025-9-3-250-256. EDN: BECKGZ
Introduction. In modern production, the requirements for reliability and durability of steel products are constantly increasing, particularly those that operate under difficult operating conditions. To address this issue, traditional methods involve forming diffusion coatings on the surfaces of these products with increased hardness and wear resistance [1, 2]. This includes carbide-type coatings obtained by diffusion saturation of steel with chromium [3], tungsten [4], molybdenum and other carbide-forming elements [5]. A significant disadvantage of this technology is that it is time-consuming, taking more than 8 hours to complete. However, it is possible to accelerate diffusion saturation by applying high-energy effects on the material, for example, plasma [6], ion plasma [7], laser [8, 9], electric spark [10], as well as heating using thermionic effects [11]. These technologies are effective, but require complex and expensive equipment. In this regard, the method of microarc surface alloying [12] has an undeniable advantage. In this process, heating and diffusion saturation of steel products occur in a metal container filled with coal powder. Heating is caused by microarcs that result from the passage of an electric current through the circuit: power source – container – coal powder – steel product. Acceleration of the diffusion saturation process is achieved by exposing the material to microarc discharges that occur between the surface of the product and the coal powder. The obvious simplicity of this technology, combined with its low energy consumption, does not require additional evidence of its advantages.
Carbide-type coatings containing molybdenum are widely used in mechanical engineering. The process of molybdenum steel saturation involves heating of chemical compounds based on molybdenum or ferromolybdenum in powders, as well as in a gaseous medium of molybdenum halides, or in melts based on sodium molybdate. This process is conducted at temperatures ranging from 1000 to 1200°С for at least six to seven hours. The use of the microarc alloying method to obtain such coatings can significantly reduce time required for this process, making it an urgent area of research [13, 14]. The main factor that determines the properties of these coatings is the presence of carbide phase particles in their structure. In this study, we aimed to investigate the features of the fine structure of the surface layer of steel after microarc molybdenum steel saturation.
Materials and Methods. Microarc molybdenum steel saturation was performed using a coating of ammonium molybdate (NH4)2MoO4 powder in an electrically conductive gel, in a volume ratio of 1:1. The coating was applied to the surface of steel 20 samples, each with a diameter of 12 mm and length of 35 mm. These samples were then immersed in a metal container containing carbon powder with a dispersion of 0.4–0.6 mm, and an electric current was passed through the source – container – coal powder – sample chain for six minutes. To achieve the desired temperature for the molybdenum plating process, a current density of 0.53 A/cm² was maintained on the surface of the samples.
After diffusion saturation, a transverse microplate was created by pouring epoxy resin into cylindrical mandrels, ensuring strict perpendicularity of the sample's surface to its longitudinal axis. The samples were then ground on abrasive papers with grain sizes ranging from P480 to P2500 and polished first with Cr2O3 oxide grade ОХА-0 according to GOST 2912–79, and finally with AM diamond paste with a 3/2 powder grain size according to GOST 25593–83. After removing any remaining paste residue with ethyl alcohol, chemical etching was performed using Rzheshotarsky reagent (a 4% solution of nitric acid in ethyl alcohol).
The microstructure was studied on a Neophot-21 microscope with a ToupCam Xcam0720P-H HDMI digital console. X-ray phase analysis was performed on an ARL X'TRA-435 diffractometer in Cu-Kα radiation. The hardness of the diffusion layer was measured with a PMT-3 microhardness meter at loads of 0.490 and 0.196 N. The molybdenum content in the layer was determined using a ZEISS CrossBeam 340 scanning electron microscope with an Oxford Instruments X-max 80 X-ray microanalyzer. The relief of the transverse section of the diffusion layer was studied on an atomic force microscope (AFM) NanoEducator in constant force mode.
Results. Metallographic analysis revealed a weakly etching coating with a thickness of 50–55 µm on the surface of the samples after microarc molybdenum steel saturation. A carbonized layer with a pearlitic structure about 200 µm thick was found under it, followed by the initial structure. The coating consisted of a dispersed ferrite-carbide mixture containing carbide inclusions up to 5 µm in size. The microhardness of the base layer was 8–9 GPa, and the carbide inclusions were up to 21 GPa (Fig. 1).
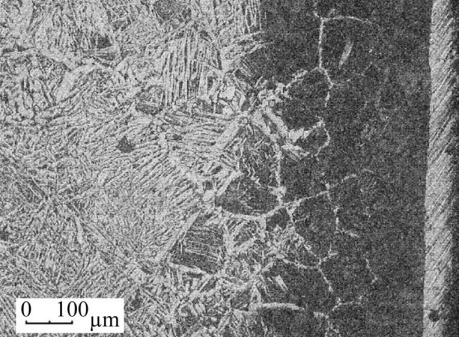
Fig. 1. Microstructure of the surface layer of steel 20 after microarc molybdenum plating
The results of measuring the mass fraction of molybdenum (Fig. 2) are presented in Table 1. From the data obtained, it could be seen that the molybdenum content at different points of the coating differed, and the coating itself had a heterogeneous composition.
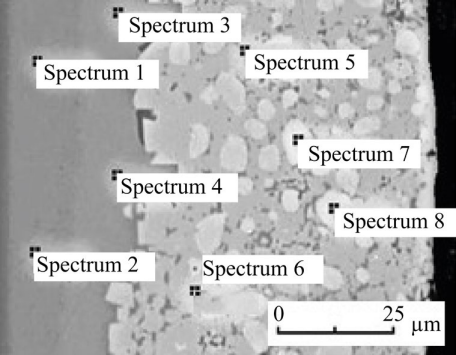
Fig. 2. Structure of the molybdenum coating in backscattered electrons
Table 1
Concentration of C diffusant at individual coating points
|
No. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
С, weight % |
– |
– |
3.1 |
3.3 |
46.8 |
47.0 |
93.9 |
94.1 |
There was no molybdenum in points 1 and 2. Next, a transition zone of a solid molybdenum solution was formed, containing about 3% Mo. The thickness of the diffusion layer was 50–55 µm. It consisted of a base (spectra 5, 6) with rounded inclusions located in it (spectra 7, 8). As can be seen from Table 1, the base contained approximately 47% Mo, and therefore it could be an intermetallic Fe3Mo2 or carbides (Fe,Mo)3C [15, 16]. The inclusions (spectra 7, 8) contained approximately 94% Mo, which corresponded to the carbide phase Mo2C [16, 17].
The formation of such carbides in the surface layer was confirmed by X-ray phase analysis (Fig. 3).
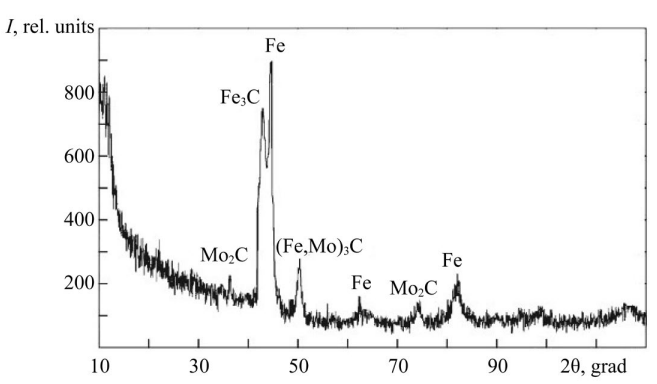
Fig. 3. Diffractogram of the molybdenum coating
High microhardness of the coating base could be explained by the formation of nanoscale carbide particles in it, which was confirmed by the results of atomic force microscopy (AFM) (Fig. 4, 5).
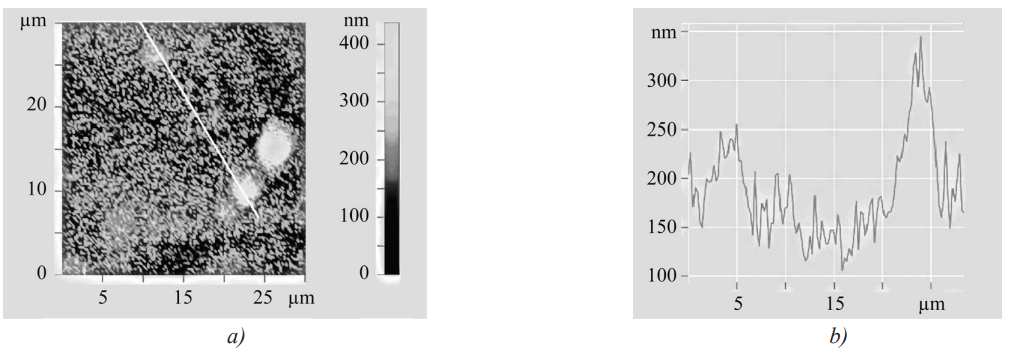
Fig. 4. Relief of the sample surface: a — section in direction 1; b — profile corresponding to this section
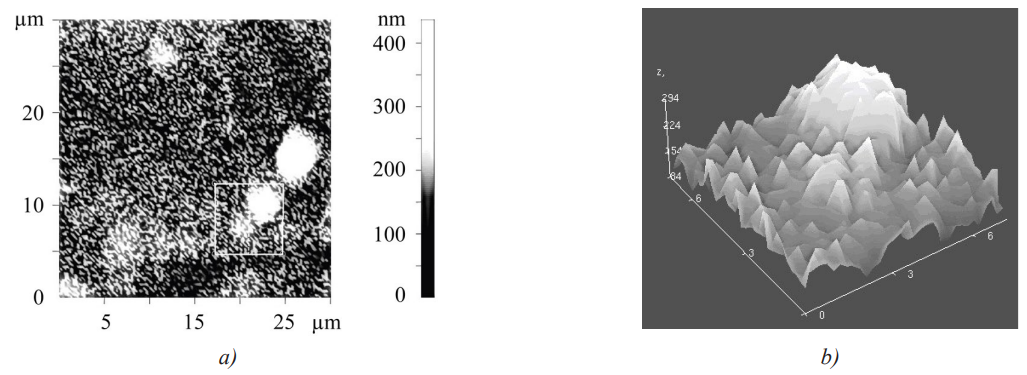
Fig. 5. Image of the steel surface obtained by the AFM method: a — 2D image; b — 3D image
Thus, the coating structure contained carbide inclusions up to 5 µm in size, as well as multiple nanoscale inclusions that protruded above the surface plane of the sample. Such inclusions had a higher hardness compared to other structural components.
To quantify the strengthening effect of these particles, it was advisable to use the hardness additivity rule, according to which HAB hardness of a two-phase alloy could be represented as the sum of the hardness HA and HB of the constituent phases A and B, taken in their volume fractions VA and VB:
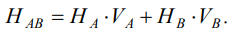 (1)
(1)
The dispersed ferrite-carbide mixture acted as phase A, the base of the diffusion layer, and nanoscale carbide inclusions acted as phase B. For the calculation according to formula (1), the following initial data were used:
HA = 3,000 MPa, HB = 23 GPa, the values of VA and VB were determined according to method [18] and assumed to be equal to: VA = 0.73; HB = 0.27. From where it was obtained: HAB = 8,400 MPa, which is consistent with the measurement results of the integral microhardness of the diffusion layer.
Discussion. The data obtained confirmed the possibility of accelerated production of a high-hardness molybdenum coating on steel using the microarc surface alloying method. With a total coating thickness of 50–55 µm, it has a complex structure in depth and consists of a dispersed ferritocarbide mixture with a microhardness of 8–9 GPa, with inclusions of relatively large particles of the carbide phase up to 5 µm in size (microhardness up to 21 GPa), and multiple nanoscale inclusions. This is followed by a carbonized layer with a pearlitic structure about 200 µm thick, which transforms into the initial structure of steel 20. A calculated assessment of the strengthening effect of such nanoscale inclusions confirmed that their presence determines the high microhardness of the coating base. It should be noted that the obtained value of the microhardness of the coating base exceeds its value, which is achieved using traditional molybdenum plating methods. It can be assumed that the formation of nanoscale inclusions of the carbide phase during microarc molybdation occurs under the influence of numerous microarc discharges that occur between the surface of the steel and the adjacent coal powder during passage of electric current. However, the physical processes taking place under such conditions require separate consideration and could be one of the areas for future research.
Conclusion. Microarc surface alloying can be used to create high-hardness coatings on steel using the method of molybdenum plating. Investigation of the fine structure of the coating has shown that it has a complex phase composition: a dispersed ferritic-carbide mixture with numerous small and nanoscale carbide inclusions, which gives the coating high microhardness. Underneath this, there is a carbonized layer with a pearlitic structure, followed by the original steel structure. The results obtained from these studies can be useful for the development of technologies to harden the surfaces of steel products such as tools and machine parts that operate in difficult conditions.
References
1. Mittemeijer EJ, Somers MAJ (eds.). Thermochemical Surface Engineering of Steels. Woodhead Publishing; 2015. 827 p.
2. Wang RJ, Qian YY, Liu J. Structural and Interfacial Analysis of WC92–Co8 Coating Deposited on Titanium Alloy by Electrospark Deposition. Applied Surface Science. 2024;228(1–4):405–409. https://doi.org/10.1016/j.apsusc.2004.01.043
3. Guryev MA, Guryev AM, Ivanov SG, Chernykh EV. Influence of the Chemical Composition of Steel on the Structure and Properties of Diffusion Coatings Obtained by Simultaneous Saturation of Structural Steels with Boron, Chromium, and Titanium. Physics of the Solid State. 2023;65(1):62–65. https://doi.org/10.1134/S1063783423700014
4. Stepanov MS, Dombrovskiy YuM. Deposition of Carbide-Type Coatings during Micro-Arc Thermodiffusion Tungstening of Steel. Materialovedenie. 2018;(1):20–25. (In Russ.)
5. Yu-Hsien Liao, Fan-Bean Wu. Microstructure Evolution and Mechanical Properties of Refractory Molybdenum-Tungsten Nitride Coatings. Surface and Coatings Technology. 2024;476:130154. https://doi.org/10.1016/j.surfcoat.2023.130154
6. Kalita VI, Komlev DI, Radyuk AA, Mikhailova AB, Demin KYu, Rumyantsev BA. Investigation of the Structure and Microhardness of Plasma Coatings Made of Austenitic Steel after Friction Treatment. Metally. 2024;(3):32–42. (In Russ.)
7. Kudryakov OV, Varavka VN, Zabiyaka IYu, Yadrets EA, Karavaev VP. Morphology and Genealogy of Structural Defects in Vacuum Ion-Plasma Coatings. Advanced Engineering Research (Rostov-on-Don). 2020;20(3):269–279 https://doi.org/10.23947/2687-1653-2020-20-3-269-279
8. Shaburova NA, Pashkeev KYu, Myasoedov VA. Comparative Analysis of Structure and Properties of Chromocobalt Coating Applied by Diffusion Saturation and Laser Surfacing. Materialovedenie. 2024;(6):12–20. (In Russ.) https://doi.org/10.31044/1684-579X-2024-0-6-12-20
9. Liexin Wu, Li Meng, Yueyue Wang, Shuhuan Zhang, Wuxia Bai, Taoyuan Ouyang, et al. Effects of Laser Surface Modification on the Adhesion Strength and Fracture Mechanism of Electroless-Plated Coatings. Surface And Coatings Technology. 2022;429:127927. https://doi.org/10.1016/j.surfcoat.2021.127927
10. Khimukhin SN, Eremina KP, Khe VK. Structure of Combined Intermetallide Electrospark Coatings on Steel 45. Metallovedenie i Termicheskaya Obrabotka Metallov. 2024;(11):20–27. (In Russ.) https://doi.org/10.30906/mitom.2024.11.20-27
11. Shaburova NA. Chromium Plating of Steel Parts Using the Thermoemission Field. Materials Physics and Mechanics. 2024;52(3):154–160. https://doi.org/10.18149/MPM.5232024_14
12. Stepanov MS, Dombrovskii YuM, Pustovoit VN. Microarc Diffusion Saturation of Steel with Carbon and Carbide-Forming Elements. Metallovedenie i Termicheskaya Obrabotka Metallov 2017;(5(743)):45–49. (In Russ.)
13. Stepanov MS, Dombrovskii YuM. Thermodynamic Analysis of Carbide Layer Formation in Steel with Microarc Saturation by Molybdenum. Steel in Translation. 2016;46(2):79–82. https://doi.org/10.3103/S0967091216020169
14. Stepanov MS, Dombrovskii YM. Microarc Molybdenum Steel Saturation Using Ammonium Molybdate. Safety of Technogenic and Natural Systems. 2024;8(4):47–53. https://doi.org/10.23947/2541-9129-2024-8-4-47-53
15. Kalin BA, Platonov PA, Tuzov YuV, Chernov II, Strombakh YaI. Structural Materials of Nuclear Engineering. In: Physical Materials Science, vol. 6. Moscow: National Research Nuclear University MEPhI; 2021. 736 p. (In Russ.)
16. Kobernik NV, Pankratov AS, Mikheev RS, Orlik AG, Sorokin SP, Petrova VV, et al. Application of Chromium Carbides in Surfacing Materials Intended for Obtaining of Abrasion Resistant Coatings. Vestnik Mashinostroeniya. 2020;(9):64–68. https://doi.org/10.36652/0042-4633-2020-9-64-68
17. Aleshin NP, Kobernik NV, Pankratov AS, Petrova VV. Thermodynamic Modeling of the Formation of Chromium Carbides in the Surfaced Metal. Vestnik Mashinostroeniya. 2020;(7):67–71. (In Russ.) https://doi.org/10.36652/0042-4633-2020-7-67-71
18. Volkov NV, Skrytny VI, Filippov VP, Yaltsev VN. Methods for Studying the Structural and Phase State of Materials. In: Physical Materials Science, vol. 3. Moscow: National Research Nuclear University MEPhI; 2021. 800 p. (In Russ.)
About the Authors
M. S. StepanovРоссия
Makar S. Stepanov - Dr. Sci. (Eng.), Professor of the Department of Quality Management, Don State Technical University.
1, Gagarin Sq., Rostov-on-Don, 344003
Scopus ID 57189900435; ResearcherID O-6959-2016
Yu. M. Dombrovskii
Россия
Yurii M. Dombrovskii - Dr. Sci. (Eng.), Professor of the Materials Science and Technology of Metals Department, Don State Technical University.
1, Gagarin Sq., Rostov-on-Don, 344003
Scopus ID 6506034790
Review
For citations:
Stepanov M.S., Dombrovskii Yu.M. Fine Steel Structure after Microarc Molybdenum Steel Saturation. Safety of Technogenic and Natural Systems. 2025;9(3):250-256. https://doi.org/10.23947/2541-9129-2025-9-3-250-256. EDN: BECKGZ
JATS XML








































